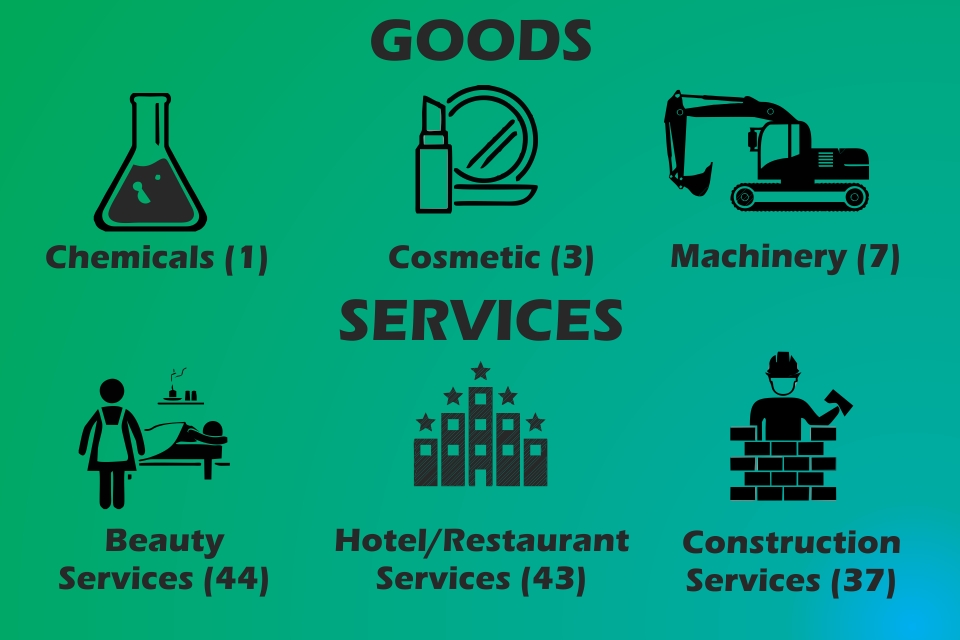
The Madrid Protocol is an international treaty that permits a trademark owner to make application of protection in the Madrid Union having 112 members and 128 countries (contracting party) in a cost effective manner. The Madrid Protocol allows trademark owner single set of fees and single application. The protocol is regulated by the International Bureau of World Intellectual Property … Read More